Details of the Drug
General Information of Drug (ID: DM5I621)
| Drug Name |
Methyldopa
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
AMD; Aldometil; Aldomin; Alphamethyldopa; Baypresol; Becanta; Dopamet; Dopamethyperpax; Dopatec; Dopegit; Dopegyt; Dopergit; Grospisk; Hyperpax; Hypolag; Medomet; Medopa; Medopal; Medopren; Methoplain; Methyldopum; Metildopa; Novomedopa; Presinol; Presolisin; Sedometil; Sembrina; Alpha medopa; Methyl dopa; Methyldopa anhydrous; Aldoril D30; Aldoril D50; Bayer 1440 L; LT00847269; MK 351; Aldomet (TN); Aldoril (TN); Alpha-Methyl dopa; Alpha-Methyldihydroxyphenylalanine; Alpha-Methyldopa; Apo-Methyldopa; Dopamet (TN); Dopegyt (TN); L-Methyl Dopa; L-Methyldopa; METHYL DOPA (SEE ALSO METHYL DOPA SESQUIHYDRATE); MK-351; Methyldopa (INN); Methyldopa (anhydrous); Methyldopum [INN-Latin]; Metildopa [INN-Spanish]; Mk. b51; Nr.C 2294; Nu-Medopa; Alpha-Methyldopa (VAN); L-alpha-Methyl DOPA; L-alpha-Methyldopa; Methyl-L-dopa; Aldoril, Dopamet, Dopegyt, Methyldopa; L-(alpha-Md); Methyldopa (L,-); Alpha-Methyl-L-3,4-dihydroxyphenylalanine; L-alpha-Methyl-3,4-dihydroxyphenylalanine; L-3-(3,4-Dihydroxyphenyl)-2-methylalanine; Levo-3-(3,4-Dihydroxyphenyl)-2-methylalanine; Alpha-Methyl-beta-(3,4-dihydroxyphenyl)-L-alanine; L(-)-beta-(3,4-Dihydroxyphenyl)-alpha-methylalanine; L-2-Amino-2-methyl-3-(3,4-dihydroxyphenyl)propionic acid; L-(-)-3-(3,4-Dihydroxyphenyl)-2-methylalanine; L-(-)-alpha-Methyl-beta-(3,4-dihydroxyphenyl)alanine; (-)-3-(3,4-Dihydroxyphenyl)-2-Methyl-L-Alanine Sesqui-Hydrate; (2S)-2-amino-3-(3,4-dihydroxyphenyl)-2-methylpropanoic acid; (S)-(-)-alpha-Methyldopa; 3-(3,4-DIHYDROXYPHENYL)-2-METHYL-L-ALANINE; 3-Hydroxy-.alpha.-methyl-L-tyrosine; 3-Hydroxy-alpha-methyl-L-tyrosine
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antihypertensive Agents
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
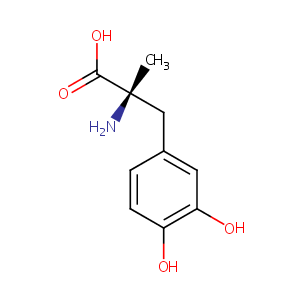 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 211.21 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -1.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Hypertension | |||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | BA00-BA04 | |||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Methyldopa (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5217). | ||||
|---|---|---|---|---|---|
| 2 | Jiao SS, Yao XQ, Liu YH, Wang QH, Zeng F, Lu JJ, Liu J, Zhu C, Shen LL, Liu CH, Wang YR, Zeng GH, Parikh A, Chen J, Liang CR, Xiang Y, Bu XL, Deng J, Li J, Xu J, Zeng YQ, Xu X, Xu HW, Zhong JH, Zhou HD, Zhou XF, Wang YJ: Edaravone alleviates Alzheimer's disease-type pathologies and cognitive deficits. Proc Natl Acad Sci U S A. 2015 Apr 21;112(16):5225-30. doi: 10.1073/pnas.1422998112. Epub 2015 Apr 6. | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | DailyMed Label: METHYLDOPA oral tablet, film coated | ||||
| 5 | BUHS RP, BECK JL, SPETH OC, SMITH JL, TRENNER NR, CANNON PJ, LARAGH JH: THE METABOLISM OF METHYLDOPA IN HYPERTENSIVE HUMAN SUBJECTS. J Pharmacol Exp Ther. 1964 Feb;143:205-14. | ||||
| 6 | Barbarino JM, Staatz CE, Venkataramanan R, Klein TE, Altman RB: PharmGKB summary: cyclosporine and tacrolimus pathways. Pharmacogenet Genomics. 2013 Oct;23(10):563-85. doi: 10.1097/FPC.0b013e328364db84. | ||||
| 7 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 8 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 9 | Gupta M, Al Khalili Y: Methyldopa . | ||||
| 10 | Centrally acting antihypertensive agents: an update. J Clin Hypertens (Greenwich). 2007 May;9(5):399-405. | ||||
| 11 | Lack of interaction between amiodarone and mexiletine in cardiac arrhythmia patients. J Clin Pharmacol. 2002 Mar;42(3):342-6. | ||||
| 12 | Platelet phenol sulfotransferase and erythrocyte catechol-O-methyltransferase activities: correlation with methyldopa metabolism. Clin Pharmacol Ther. 1984 Jan;35(1):55-63. | ||||
| 13 | Identification, characterization, and ontogenic study of a catechol O-methyltransferase from zebrafish. Aquat Toxicol. 2011 Mar;102(1-2):18-23. | ||||
| 14 | Molecular mechanisms controlling the rate and specificity of catechol O-methylation by human soluble catechol O-methyltransferase. Mol Pharmacol. 2001 Feb;59(2):393-402. doi: 10.1124/mol.59.2.393. | ||||
| 15 | Inhibition of human glutathione S-transferases by dopamine, alpha-methyldopa and their 5-S-glutathionyl conjugates. Chem Biol Interact. 1994 Jan;90(1):87-99. | ||||
| 16 | Neonatal effects of methyldopa therapy in pregnancy hypertension. Acta Paediatr Hung. 1991;31(1):53-65. | ||||
| 17 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 18 | Darcy PF, Griffin JP "Interactions with drugs used in the treatment of depressive illness." Adverse Drug React Toxicol Rev 14 (1995): 211-31. [PMID: 8845455] | ||||
| 19 | Gachalyi B, Tornyossy, Vas A, Kaldor A "Effect of alphamethyldopa on the half-lives of antipyrine, tolbutamide and D-glucaric acid excretion in man." Int J Clin Pharmacol Ther Toxicol 18 (1980): 133-5. [PMID: 6103880] | ||||
| 20 | Product Information. Sirturo (bedaquiline). Janssen Pharmaceuticals, Titusville, NJ. | ||||
| 21 | Aronowitz JS, Chakos MH, Safferman AZ, Lieberman JA "Syncope associated with the combination of clozapine and enalapril." J Clin Psychopharmacol 14 (1994): 429-30. [PMID: 7884028] | ||||
| 22 | Product Information. Turalio (pexidartinib). Daiichi Sankyo, Inc., Parsippany, NJ. | ||||
| 23 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 24 | Product Information. Adcetris (brentuximab vedotin). Seattle Genetics Inc, Bothell, WA. | ||||
| 25 | Product Information. Kynamro (mipomersen). Genzyme Corporation, Cambridge, MA. | ||||
| 26 | Canadian Pharmacists Association. | ||||
| 27 | Product Information. Juxtapid (lomitapide). Aegerion Pharmaceuticals Inc, Cambridge, MA. | ||||
| 28 | Campbell N, Paddock V, Sundaram R "Alteration of methyldopa absorption, metabolism, and blood pressure control caused by ferrous sulfate and ferrous gluconate." Clin Pharmacol Ther 43 (1988): 381-6. [PMID: 3356082] | ||||
| 29 | Al-Nawakil C, Willems L, Mauprivez C, et.al "Successful treatment of l-asparaginase-induced severe acute hepatotoxicity using mitochondrial cofactors." Leuk Lymphoma 55 (2014): 1670-4. [PMID: 24090500] | ||||
| 30 | Product Information. Zydelig (idelalisib). Gilead Sciences, Foster City, CA. | ||||
| 31 | Product Information. Zulresso (brexanolone). Sage Therapeutics, Inc., Cambridge, MA. | ||||
| 32 | Product Information. Reyvow (lasmiditan). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 33 | Ban TA "Drug interactions with psychoactive drugs." Dis Nerv Syst 36 (1975): 164-6. [PMID: 1116424] | ||||
| 34 | Illi A, Sundberg S, Ojala-Karlsson P, Korhonen P, Scheinin M, Gordin A "The effect of entacapone on the disposition and hemodynamic effects of intravenous isoproterenol and epinephrine." Clin Pharmacol Ther 58 (1995): 221-7. [PMID: 7648772] | ||||
| 35 | Product Information. Clozaril (clozapine). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
